Molecular Solar Thermal Energy Storage System [MOST]
According to the 2015 United Nations Climate Change Conference (COP21) Paris agreement on climate change, global emissions must be reduced by 60% prior to 2050.1 The energy consumption is, however, predicted to double in the next 40 years due to an increasing world population.2 For this reason, it is prudent to explore other sustainable energy sources, in addition to electricity generated by either wind power or solar cells.
This promising research was developed by a group of scientists in the Department of Chemistry and Chemical Engineering, Chalmers University of Technology, at Gothenburg, Sweden.
Abstract
The development of solar energy can potentially meet the growing requirements for a global energy system beyond fossil fuels, but necessitates new scalable technologies for solar energy storage. One approach is the development of energy storage systems based on molecular photoswitches, so-called molecular solar thermal energy storage (MOST). Successful outdoor testing shows proof of concept and illustrates that future implementation is feasible. The mechanism of the catalytic back reaction is modelled using density functional theory (DFT) calculations rationalizing the experimental observations.
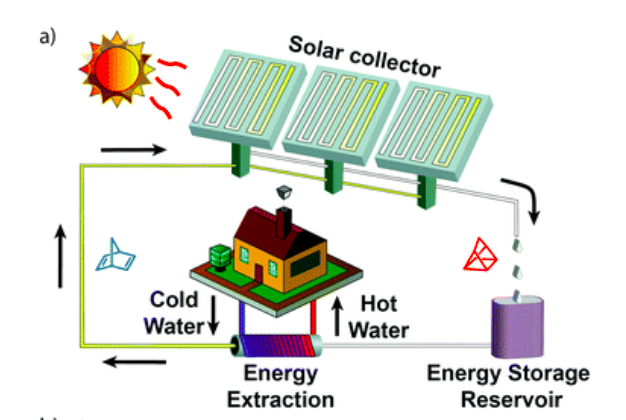
Thermal energy can be used for a broad range of applications such as domestic heating, industrial process heating and in thermal power processes. One promising way to store solar thermal energy is so-called molecular solar thermal (MOST) energy storage systems, where a photoswitchable molecule absorbs sunlight and undergoes a chemical isomerization to a metastable high energy species. Here we present an optimized MOST system (providing a high energy density of up to 0.4 MJ kg−1), which can store solar energy for a month at room temperature and release the thermochemical energy “on demand” in a closed energy storage cycle. In addition to a full photophysical characterization, solar energy capture of the present system is experimentally demonstrated by flowing the MOST system through an outdoor solar collector (≈900 cm2 irradiated area). Moreover, catalyst systems were identified and integrated into an energy extraction device leading to high temperature gradients of up to 63 °C (83 °C measured temperature) with a short temperature ramp time of only a few minutes. The underlying step-by-step mechanism of the catalytic reaction is modelled in detail using quantum chemistry calculations, successfully rationalizing the experimental observations.
Full text of this paper is here ![]()

Leave a Reply
You must be logged in to post a comment.